What is process validation?
Actually, process validation is key analytical process for ensuring process reliability and capability of consistent to product quality. These procedure defines the values of criteria based on evaluation of data collected from stage of production. Which may help to confirm that the process will consistently lead to the expected results. Another hand, process re-validation is performed to verify ascertain their effects of modification or new introduced elements in the process. It may impact product quality as changes are made in the operating procedures and manufacturing processes.
Overview
Whenever a real cycle process, material, machine or any other problem that affects the quality of the product by any major changes, it needs to be fixed. The Process validation and re-validation is successive used on actual conditions. It will be done to measure required criteria which establish sequence of tasks for maintain consistent of quality.
As you know, process validation and re-validation study are conducted to ensure that the process is reliable for the quality and consistency of the product. Sometime due to changes of customer expectations and behavior, changes in the products are made. More frequent management also change some processes due to costing, lean and reliability of processes. Whatever the cause but need to take more attentions for the impacts on real production, quality and consistence.
How to choose right method of validation and re-validation study.
Choose the method for validation of process according to internal process, process parameters, mechanism and rhythm of workplace eco-system. Because any validation process method which you choose, have to confirm that the analytical procedure empowered for specific test is suitable for its intended use. Just an example, some product measurements should possible with digital micro-meters only, if you use ordinary micro-meter can display variations in two results.
How to do process validation study
Basic concept is coming from R & R study that same as takes a trial one to five for the checking the all required criteria, but here some things are different or little awkward for the process validations.
Step 01 – Preparation and conducting validation
In The Process validation, there about 10 standard samples are takes for the sampling and inspect for the validations that are also match with all the parameters as cross examine that all the process is on ease. There can be various process’s parameters are match with single sample on first trial that are pass from all the machine parameter to ensure the single sample result and then same will be application for the others.
That means is so many trials are needed to show the real picture of the results. If the process’s parameters are more than 7 and taken the trial five, that means about thirty-five result you will got with single cycles. It’s very tough for the process validations, but still here are not end the process validations, it should be analyzing the information from the results.
Step 02 – Analyzing Obtained Results
All obtained results should take for re-checking for the cross examinations. Analyst can analyze obtained results to confirm with acceptable criteria is matching with results according to standards.
One of the simple or initial method is: Re-check measurements with product by same instrument, conditions, machine and procedure. Verify the variation percentage should up to acceptable level. During the re-check process, analyst must ensure the parameters, eco-condition (such as humidity), and resources are used are reliable, calibrated and it used in validation.
Step 03 – Reporting and reviews
In this part of the stage of process validation, all the detailed information is defining in a document. A document describes the detailed information about the final quote as results of validation of process. Process validation report is evident all the outcomes of parameters are achieved as standards or unstained process are identified. These report is helps to management, to determine for the sustain these process or further changes are requiring to meet standards.
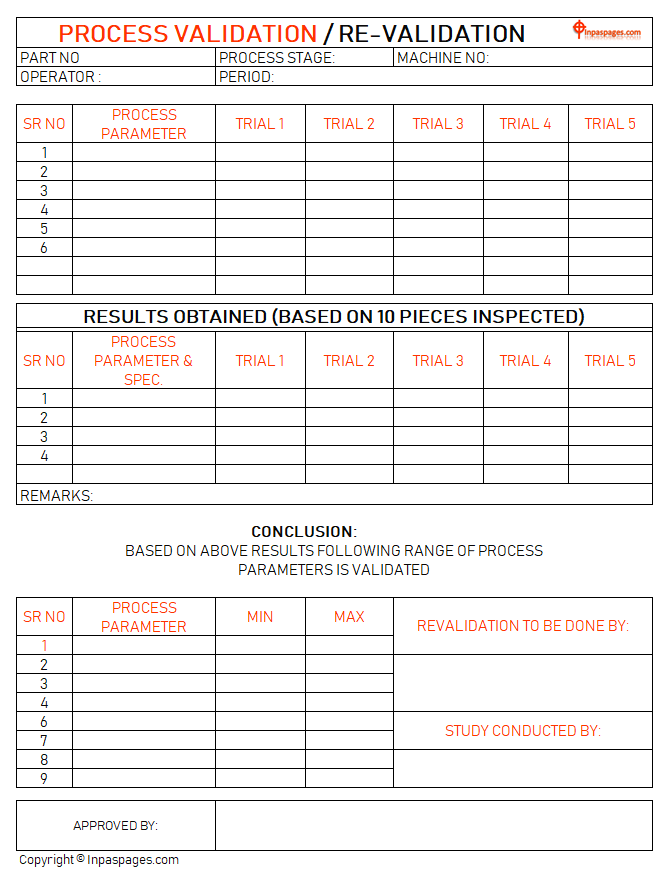
Conclusion
To do the conclusion for the based on results that obtained by both the experiments or process that done for process validation and re-validation study. In the conclusion are show the parameters are valid or not? In the process validation and re-validations are also gives a result with the future support. How? Process parameters are set up for the both process are records with the maximum level of the trial validations and minimum level from trail validations are used for process standard parameter to maintain tolerance of parameter to improved perfect product.
Pingback: What is Process Analysis?
Pingback: Equipment Log book | Instrument Log book : ISO 17025 - Technical Requirement